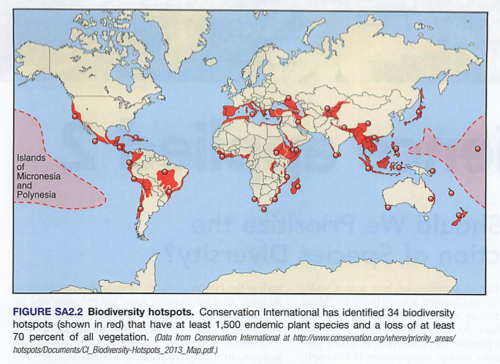
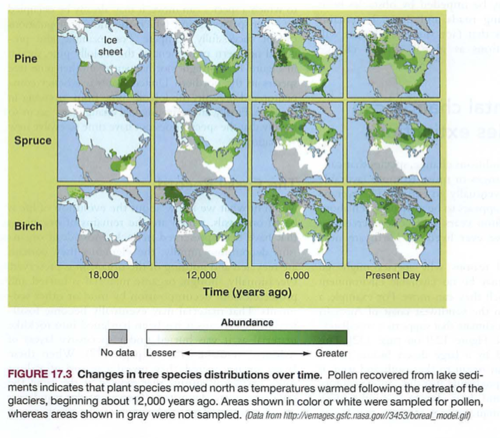
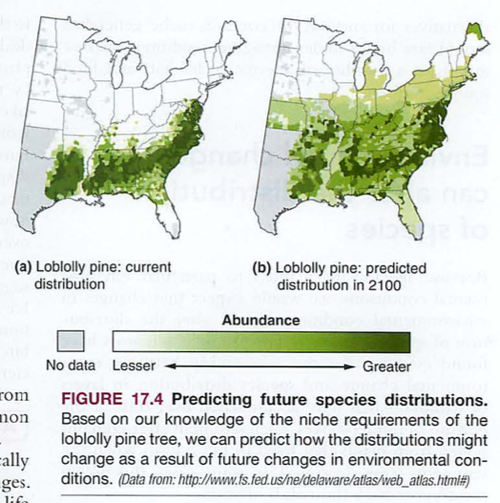
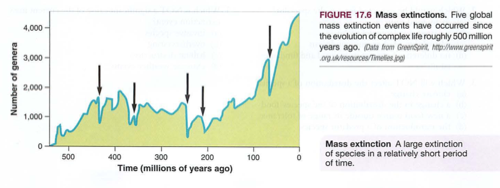
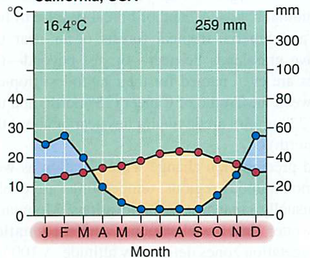
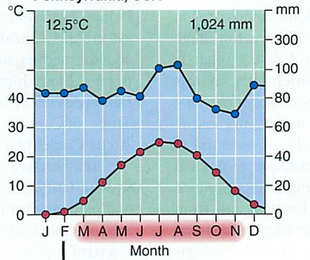
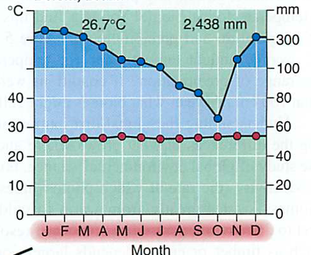
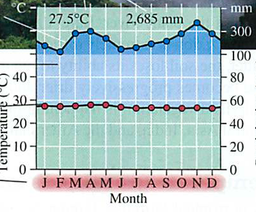
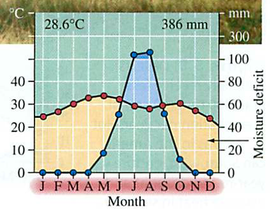
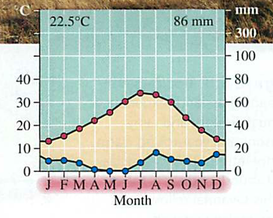
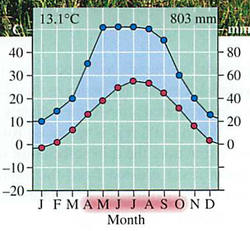
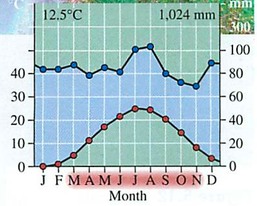
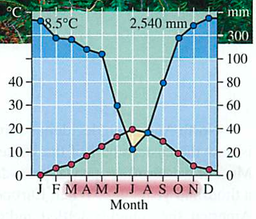
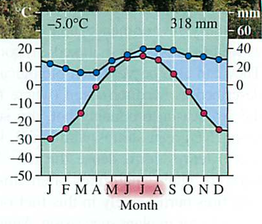
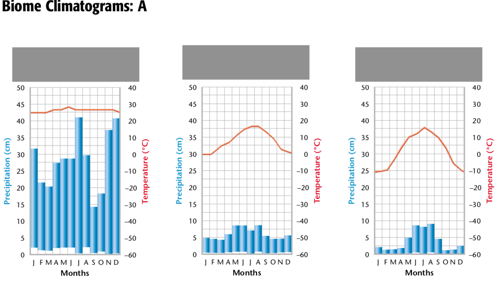
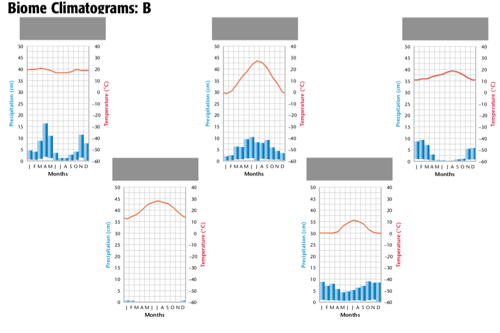
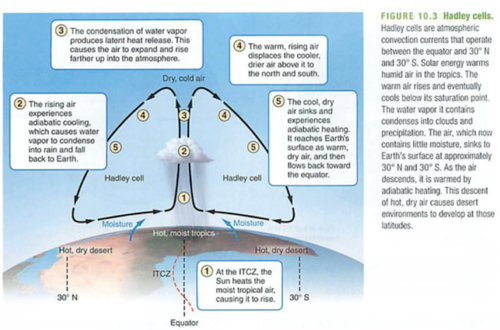
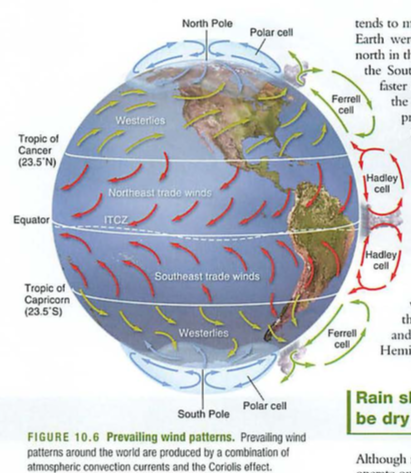
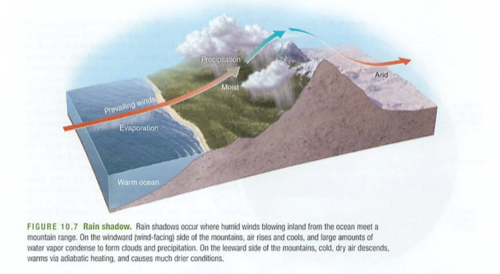
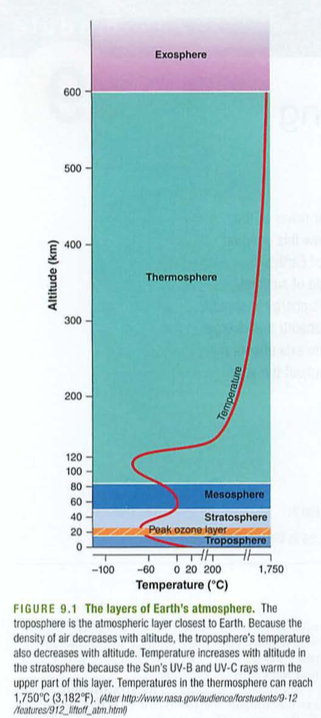
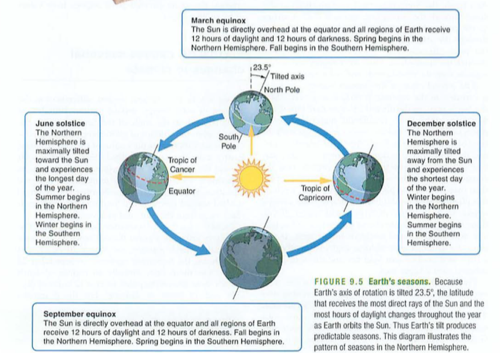
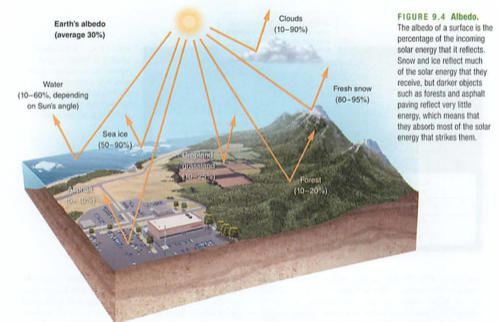
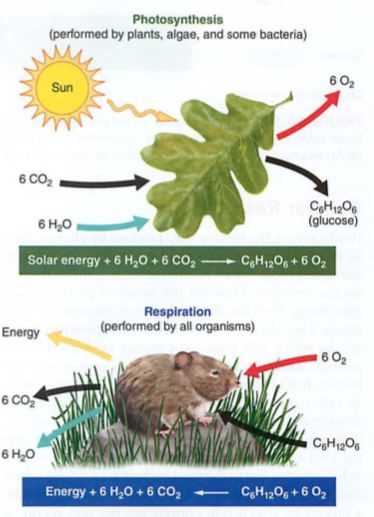
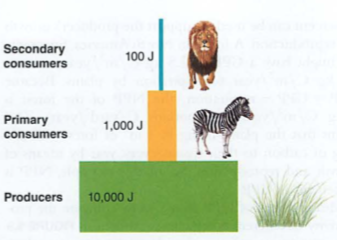
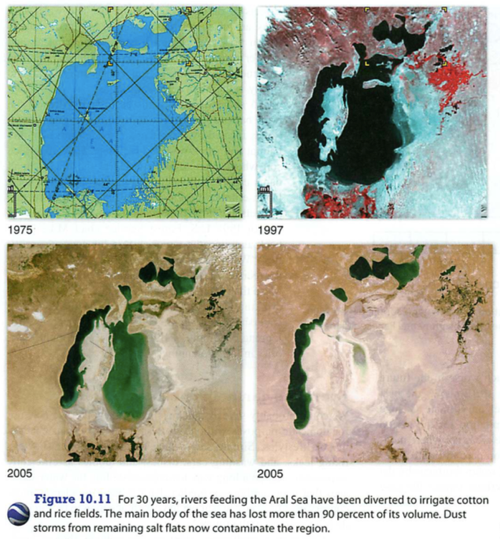
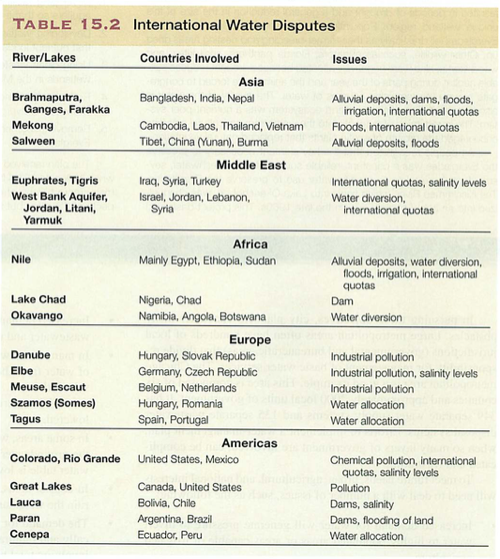
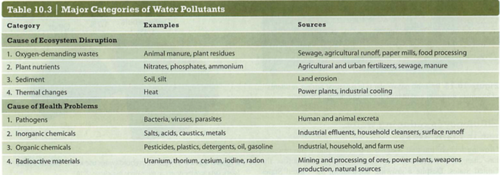
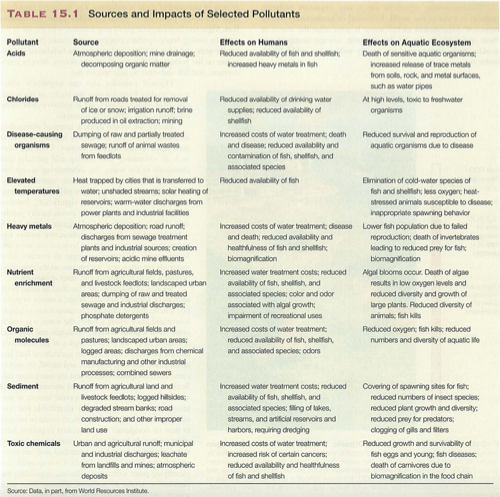
Module 6: Movement of energy
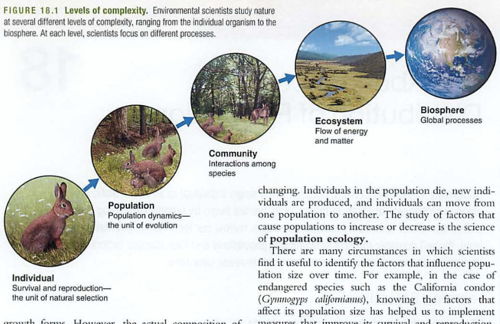
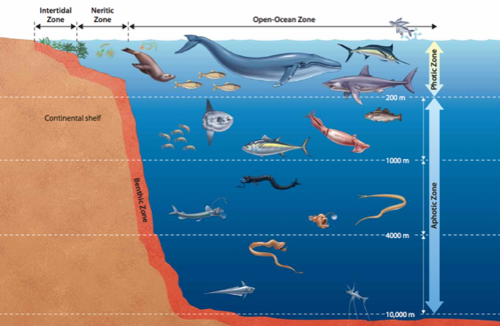
Biosphere-all life
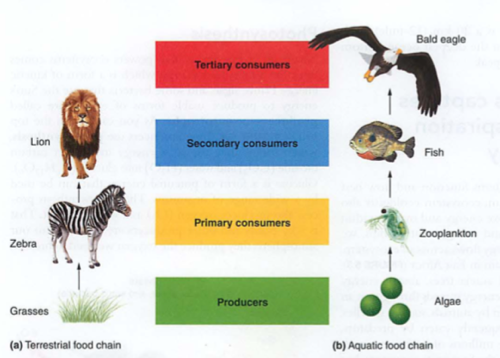
Producer (also primary producer) gets energy from sun or heat directly, also known as autotroph
example: grass
Photosynthesis: CO2 + water + energy (heat or light) -> sugars (CHO)
Reverse process is respiration: Sugars + O2 -> energy + CO2 + water
Anaerobic respiration (e.g. bacteria) don't use O2, less efficient, older primitive source of energy (pre-oxygen)
Consumer (heterotroph) consumes other primary autotrophs
- Primary consumers eat plants (e.g. herbivores)
- Secondary consumers eat them (carnivores)
- Tertiary consumers eat other carnivores (algae->zooplankton->fish->eagles)
Trophe = "nourishment"
Trophic levels = food levels, see also food web
- Scavenger-eats dead stuff
- Detritovore-eats dead stuff that is decaying
- Decomposers-break down into basic elements
GPP gross primary productivity= Total amount of solar energy into the system: like gross income in a business
e.g. all of the money coming into your business
NPP net primary productivity = GPP minus respiration (think of Maui onions):
gross income-expenses=net income
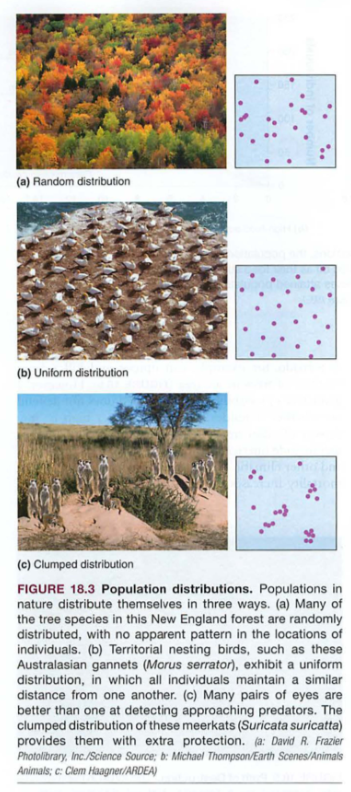
Biomass-total mass of all living things in an ecosystem-odd fact: rain forest soil is the lowest in nutrients and biomass-why?
Standing crop-total plant biomass (e.g. forest)
Efficiency is low (around 10%)
Trophic pyramid: 10x reduction in energy for each level (why?)-vegetarians vs. meat eaters
So, carnivores (us, wolves) are eating harvesting devices (cows) for autotrophs (grasses):
Not very efficient, unless you can't digest grass, or it takes too much energy to walk around all day. This IS, however a good argument for global sustainable survival, as the sun will not go away soon, and as long as we have arable (farmable) land to grow plants, we could survive with a global population of 8-10 billion.
But we'd need access to fresh water...
Recall: energy->water->food->culture
 |
|
Click for full-size image |
Module 7: Movement of Matter
Biogeochemical cycles: bio=life geo = minerals chemical = chemical cycle = something that rotates
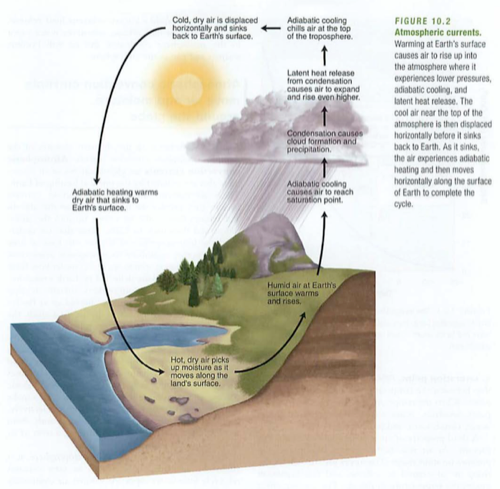
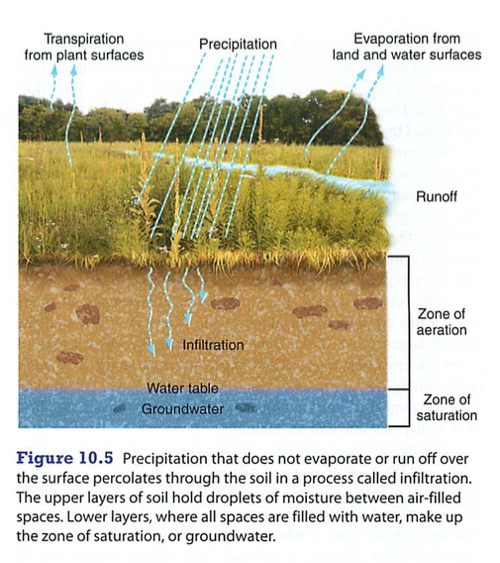
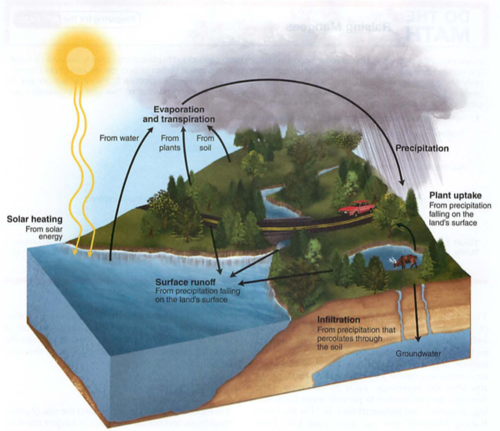
Hydrologic (water) cycle:
Heat provides energy for evaporation,
Plants secrete water as transpiration,
Condensation is collection of water vapor to drops,
Precipitation is the falling of these drops as rain, snow, hail etc.
ETO evapotranspiration: amount of water moving through an ecosystem, usually plants.
Farmers need to know EtO to know how much water to supply to their crops.
Eto depends on sunlight, humidity, wind and temperature (think of how these impact transpiration)
 |
|
Click for full-size image |
Runoff-just like it sounds
You will need to know about "transit time" which is the time it takes rain to reach a water body after hitting the ground
Why is this crucial?
(you will see this mentioned in the Poisoned Waters video this weekend)
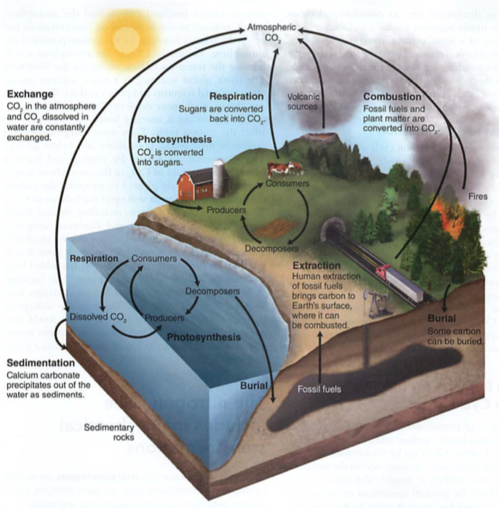
Carbon cycle: photosynthesis, respiration, exchange, sedimentation, burial, extraction, combustion
air<->water<->land
photosynthesis-CO2 to sugar (air to plant structures or fruits-e.g. corn)
respiration-sugar to CO2
acidification or exchange-HCO3 (carbonic acid) e.g. seawater changing pH, remember?
sedimentation-CaCO3 (seashells, limestone) most efficient long term carbon sink (solids are dense)
burial-just like it sounds, oil, coal, nat gas
extraction-mining fossil fuels
combustion-burning fossil fuels with oxygen to release CO2
 |
|
Click for full-size image |
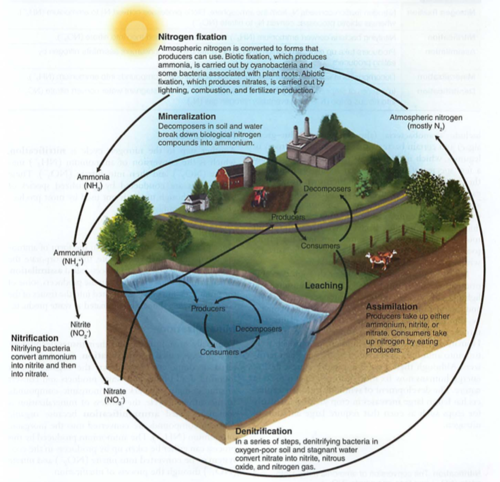
Nitrogen cycle:
6 macronutrients needed by plants: N,P,K, Ca, Mg, S (sulfur becomes part of Methionine, an amino acid)
NPK from fertilizers
N is a limiting nutrient
N fixation is when you can't get Nitrogen off your mind
quiz
- Define N, P, K and how each impacts plants
- Define evapotranspiration, condensation and precipitation
- Which biogeochemical cycle only has a solid phase in the environment?
- What element replaces oxygen in chemosynthesis?
It also means bringing N2 gas from the atmosphere into the biome, biotically or abiotically (without life)
abiotic: lightning or burning fossil fuels (high temps) direct to nitrate (NO3) (that interesting smell after rainstorms)
biotic: nitrogen fixing bacteria: N2 ->NO2 ->NO3 ->NH3 ->NH4 ion (used by producers, it is aqueous)
commercial N fixation: petrochemicals to form fertilizers (NH3, anhydrous ammonia, ammonium nitrate NH4NO3, which is used in ANFO bombs)
nitrification: NH4+ ->NO2- ->NO3-
nitrite kills bacteria (used in preserving meats), but nitrate is a good plant fertilizer (ANFO bombs)
assimilation: producing amino acids and then proteins (chains of AA)
Dead stuff: mineralization or ammonification (dead fish smell: NH4+ and amines)
Vitamins = "vital amines"
denitrification: NO3- goes to N2O to N2 gas (anaerobic bacteria, swamps)
Leaching: washing N out of soil
 |
|
Click for full-size image |
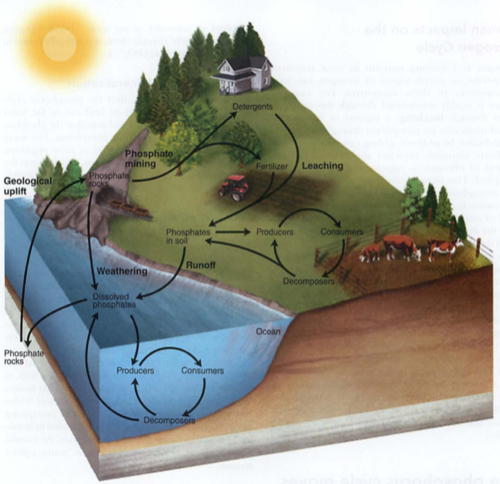
Phosphorus cycle:
Mainly rocks, between land and water
ONLY SOLIDS, NO LIQUID OR GAS PHASE
biotic: animals uptake PO4--- (see also phosphoric acid H3PO4), turn it into bones, teeth as CaPO4, then back to soil
abiotic: phosphate sediments in ocean-> become rocks, erosion on land dissolves into watershed
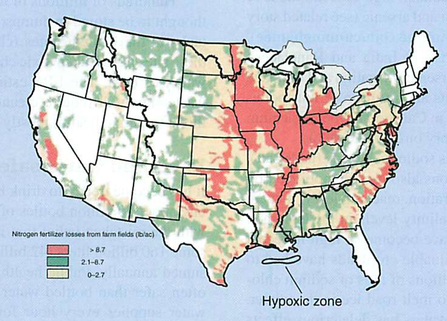
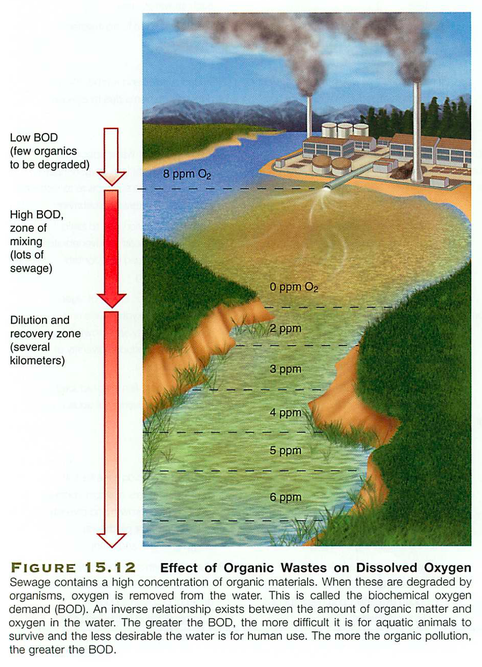
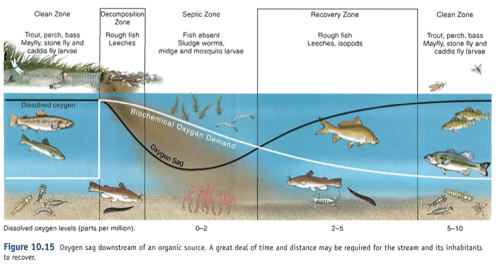
Humans: phosphate detergents, fertilizers (Dead zones)
Algal bloom: PO4 -> lots of algae on surface (light) -> these die, fall to the bottom, and as they decompose, they take all O2 from the water (hypoxia)
Arsenic in bananas---why we cannot use greywater for irrigation
 |
|
Click for full-size image |
Ca, Mg and K: dust (Kauai dependent on Gobi desert dust for K)
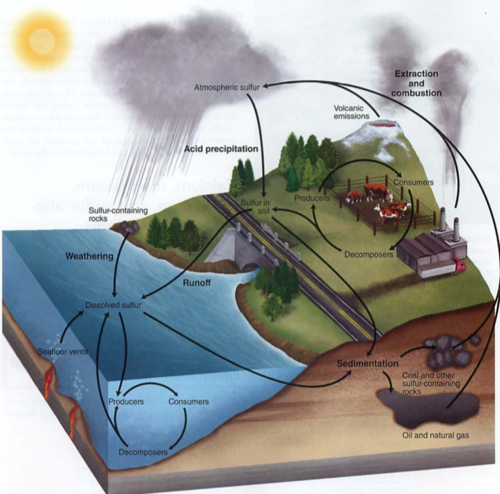
Sulfur cycle:
rocks -> SO4, can become part of methionine
See also SO2 (vog, acid rain, pollution)
 |
|
Click for full-size image |
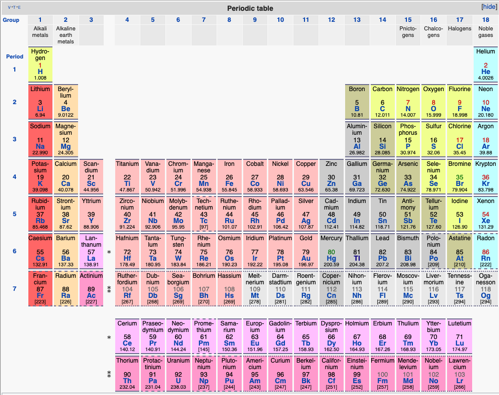
Here's something interesting:
Find Sulfur below:
 |
|
Click for full-size image |
Ok, now find Oxygen.
Hydrothermal vents use sulfur instead of oxygen for a thermal (heat) version of photosynthesis called chemosynthesis
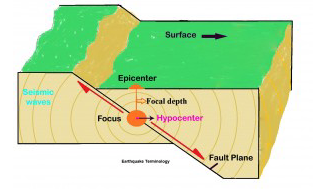
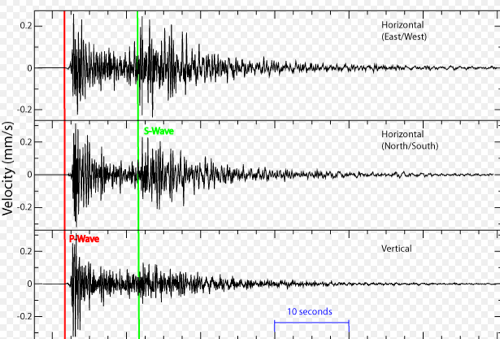
Module 8: Response to disturbances
Recall negative feedback (stable ships, relationships): greater the disturbance, the greater the restoring force (pendulums too)
Resistance is how hard it is to move the pendulum (ecosystem) away from center
Resilience is how fast the pendulum (ecosystem) returns to normal
Biodiversity and disturbances:
This is strange stuff:
Rare disturbances = competition, so only a few species dominate
Common disturbances = only fast reproducers survive, so low biodiversity
Intermediate disturbances = highest biodiversity
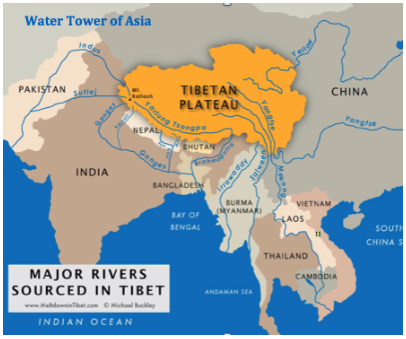
Watershed: drainage basin usually leading to a large body of water (Chesapeake watershed is huge):
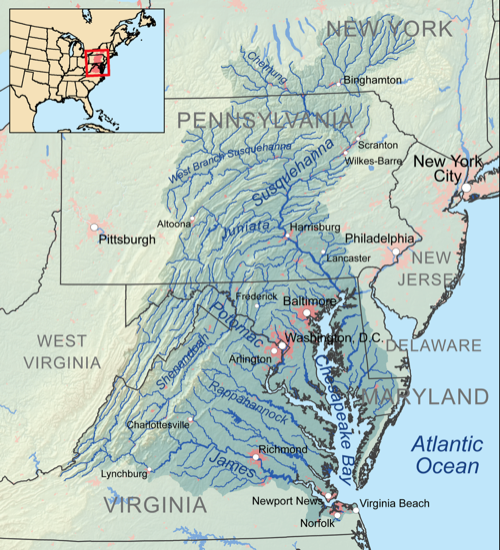 |
|
Click for full-size image |
How do you think this impacts the water quality, phosphates, nitrates and silt in the Chesapeake bay?
Why are we not allowed above the fences behind Pu'u La'e La'e?
On Oahu, the watershed is guarded by a barbed wire fence-why?
How are the Himalayas acting as a watershed?
Poisoned waters:
Video:
Weblog page:
On campus try these:
quiz
- why is the soil on Kauai so poor, and how does it get its Potassium?
- how does the size of the Cheseapeake watershed impact eutrophication in the Chesapeake bay?
- what is the difference between resistance and resilience in natural systems?
- why do rotting fish smell so bad?
Summary notes:
Systems
- systems are usually connected, exchange matter and/or energy
- main energy source is our sun, or past suns (e.g. uranium)
- feedback loops (again)
- spheres: litho=stone, bio=living, hydro=water, atmo=above
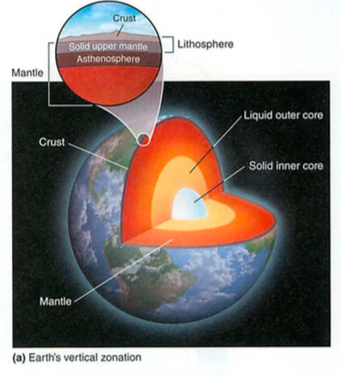
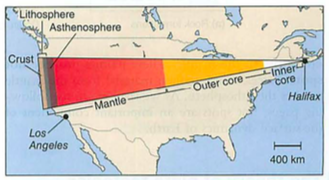
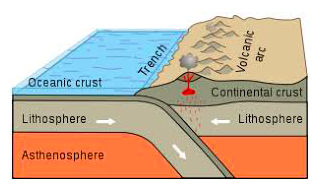
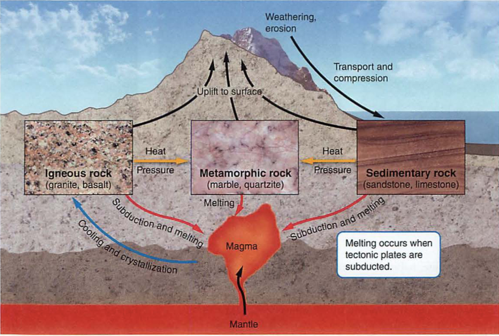
- geosphere: crust-mantle-core-inner core (spins, no way!)
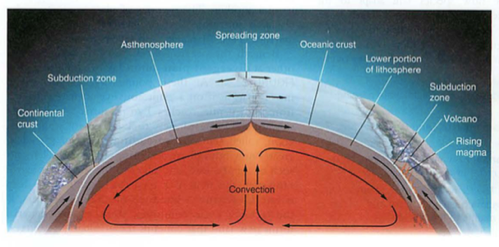
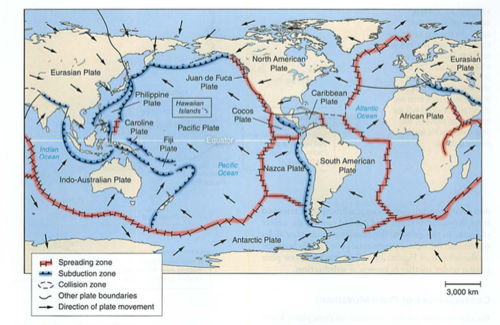
- plates-who thought this up? need to understand convection
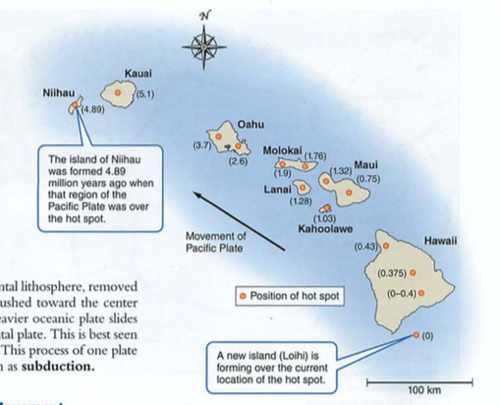
- where are oldest parts of crust? trick question
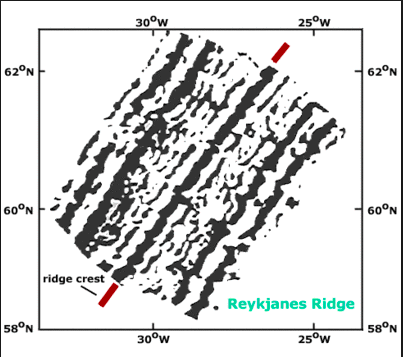
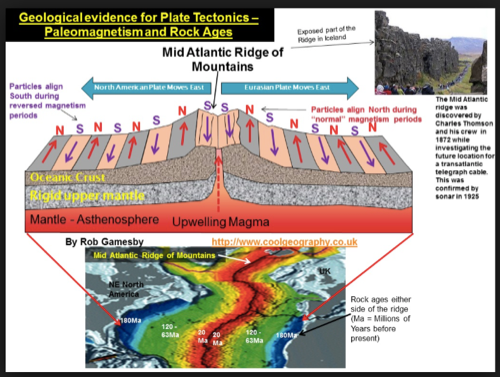
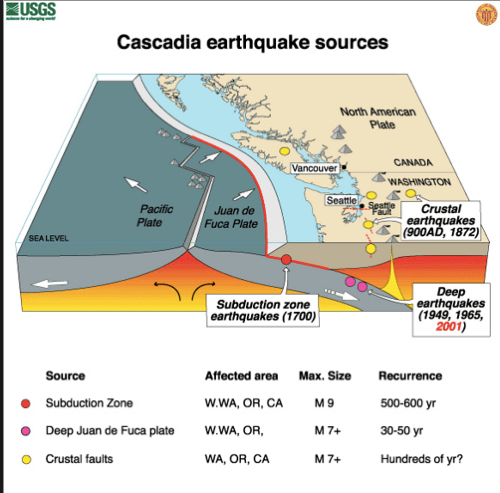
- subduction zones, mid atlantic ridges (atlantis?)
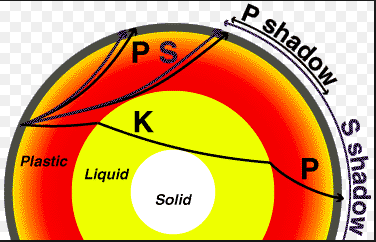
- eq: deep near subduction zones, shallow in transform faults
- mid atlantic ridge: how do we know this? submarines
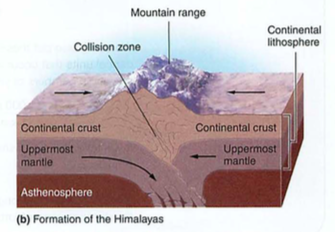
- himalayas-how? matterhorn-what?
- water cycle: evaporation and transpiration (eTO)
- precipitation-condensation is first (condensation nuclei, e.g. vog)
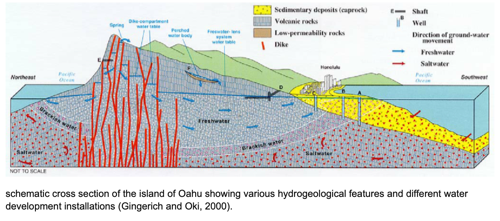
- aquifers-underground lakes, low turnover rate (pesticides) Oglalla
- water can be ground water: aquifer, water table, water lens or surface water
Cycles
- conservation of matter (neglect E=mc2)
- nutrient cycles: carbon, oxygen, phosphorus, nitrogen COPN
- primary producers-photosynthesis: CO2, water and light/heat
- create sugar as stored food, structural element
- consumers-eat primary producers, decomposers break down waste/detritus
- cellular respiration (flowers in hospitals) at night
- carbon also in HCO3- ion (seawater) and CaCO3 (seashells, limestone)
- greenhouse effect from CO2 captured as plants->oil/coal "carbon bank"
- phosphorus: rocks and water (stone and sea), erosion, very limited supply in biosphere (Waterloo bones)
- P only absorbed in aqueous form (aq)
- too much = eutrophication (also nitrogen) see dead zones
- eutrophication: too much of a good thing, when algae dies, decomposes, creates hypoxic zones
- aerobic vs. anaerobic bacteria-wounds
- nitrogen cycle-NPK
- N2 to life: lightning and bacteria (N2 to ammonia NH3 then to NO2 and NO3 (plants absorb NO3)
- denitrifying bacteria change NO2 back to N2 (gas)
- Haber process
- See figure 3.30-nitrogen uptake
- golf courses-Mauna Kea, iBoat