Water:
Key points:
Amphoteric: both an acid and a base, so pH is 7.0 (between 0 and 14), so pOH is also 7 (cool!)
Cohesion: recall the weak hydrogen bonds, these make water want to "hold hands": COhesion (think of pilot and copilot)
Adhesion: water likes to stick to other surfaces, just like adhesive tape
Capillary action: both of these working together-think of the meniscus in chemistry (U shaped dip)
Water has a U shaped meniscus, because adhesion is stronger than cohesion
Liquid mercury metal has an inverted U meniscus, because cohesion is stronger than adhesion
Why is this important?
Long hair and tall trees: both require fibers (adhesion) close together (cohesion) so the meniscus "climbs" up the tree
Surface tension: cohesion at work-think of water striders (evil trick: one drop of detergent makes all water striders fall into the pond)
Water molecules "holding hands" means insects can sit on the water surface.
Soaps and detergents break down surface tension, making water "wetter"
Why is this important?
Soaps are surfactants, and impact premature babies lungs (no surfactant, so the surface tension is so strong they cannot inflate their lungs). Vaping also dissolves this lung surfactant. Same with folks who breathe in gas fumes (like siphoning) who die from chemical pneumonia.
Osmosis: not in this chapter, but water anyway:
Water always follows salt or sugar, anything that is more concentrated.
Root hairs have sugar in them (why we chew on sugar cane), so the water in the soil is pulled into the roots.
Minerals or salts in the soil will prevent this, so we get "desertification" from using mineral rich well water on crops.
Romans also poured salt on fields of enemies so they could never live there, look up "salting the earth"
Acids and bases: the amount of hydrogen in an aqueous (water) solution
Very small numbers, so we use log10 for them:
[H+] = 1 ee-4 would be an acid with pH 4.0 and pOH of 12
pH and pOH always add to 14
[H+] =1 ee -0 is the strongest acid (pH 0), [H+] =1 ee -14 is the weakest acid and strongest base (pH 14)
Freezing point/melting point: can be depressed by adding other materials (salt, alcohol, antifreeze)
Boiling point: same deal, can be raised by adding salt (cooking pasta) or adding pressure (warm tea at altitude)
Solubility of gases: cool water can hold more dissolved gases, like oxygen or CO2 (perrier)
This is very important in thermal pollution, where heat from power plants starves fish of O2, same thing with dead zones like Chesapeake bay (more on this soon).
hint: don't ask for ice in your drinks, they must be cooled already to dissolve the carbonation
Chemical reactions: usually involve energy of some sort (heat, light-explosions, burning), and change of mass
Conservation of mass: matter never goes away in chemical reactions (it DOES in nuclear reactions)
Organic chem: any chemistry involving Carbon (why?)
Inorganic=either no carbon, or bound carbon (C02, like in carbon dioxide)
All life on our planet revolves around carbon in some form, so we use the term organic to describe this chemistry, usually C-H or C-C bonds.
Look on the periodic table below Carbon, we could be Silicon, but we'd have to be lava creatures since the energy needed for chemical reactions would be higher.
n.b. some thermal creatures use thermosynthesis instead of photosynthesis, using sulfur instead of oxygen (look again at the periodic table)
Good example: alcohols-always involve some form of carbon and an OH group, but they are NOT bases, weird rules here...
One can live on alcohols in some cases (alcoholics)
methanol: CH3OH (this is what killed people in prohibition by making "bathtub gin", decomposes into ant poison)
ethanol: C2H5OH (you know this as the type that gets one drunk-poisonous in large doses)
propanol: C3H7OH (you know this as isopropyl alcohol, what they use before they give you a shot)
All organic chem follows these meth (1 carbon), eth (2 carbons), pro (3) , but (4), pent (5) prefixes, many like in geometry.
Another example: octane (in gasoline)
How many carbons?
Much more on this later...
Food!
Three main groups:
Proteins: always include Nitrogen
carbohydrates: CHO in chains (starches) or simple (sugars)
fats: CHO again, but in a special branch structure (glycol) that is good for energy storage and insulation (whales)
Proteins: complex molecules of CHO and N. Look up amino acids, note the common structure.
Now look up the amino acid methionine. What element does it contain as well? Why do rotten eggs, swamps (and Kilauea volcano) stink?
CHO=carbohydrates (clever name), usually in a chain, short chains are sugars (used for fuel), longer ones are starches and can be used for structures (e.g. cellulose in plants) or pasta...
smallest: sugars, all end in -ose (glucose, sucrose) LOOK THESE UP, CHECK OUT THEIR MOLECULE SHAPE
glucose is a "monosaccharide" created by photosynthesis (next chapter)
longer chains: starches (rice, pasta) slowly digested (see diabetics, and glycemic index)
structural CHO: cellulose-little boxes with goo inside, need enzymes to break these down (cows)
ENZYMES ALL END IN -ASE
Fats/lipids: same chemical structure as CHO, but built along a glycol (alcohol) backbone.
If the fats have long carbon chains with only single bonds, they are saturated (lots of Hydrogen atoms) and can hold together (e.g. animal fat)
If the long chains have double bonds and don't fit together, they melt easier (e.g. oils) and are called "unsaturated", usually better for your health.
n.b. McDonalds® got into real hot water a while ago for frying all of their stuff in "supersaturated fats". Ugh...
Energy
Energy is the ability to do work (heard that before?)
Units are joules ("jowles in England), and a few others (calories, Calories, BTU, kWh)
Power is how fast you can do the work (climbing stairs or running up stairs), so Power = work/time
Units are Watts (joules per second or j/s) among others
Energy->Joules (work~amount of water)
Power->Watts (how fast the work is done~flow)
demo: walking/running upstairs-----------------
KE/PE: Kinetic and potential energy
PE: chemical bonds, height, spring
KE: motion, freewheel, flowing air/water
temp: KE=1/2mv^2 (macro level)
molecular level:
KE=3/2kT, so T prop to v^2 of molecules
EMR: shorter wavelengths more energy e.g. UV, X-rays
Light is one form of EMR or electromagnetic radiation (needs no medium, so we get light from the sun through the vacuum of space)
What you need to know: EMR has higher energy with higher frequency (e.g. ultraviolet light damages DNA, infrared heat can only burn)
See visible spectrum:
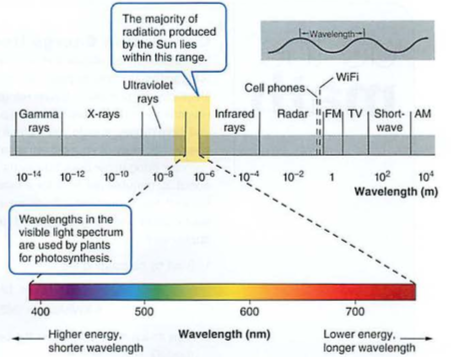
Energy can be potential (ability to do work) like altitude or chemical bonds or kinetic (see Kinesias in Lysistrata), the energy of motion or heat (molecules in motion, KE = 3/2kT)
Temperature is not heat, but the average speed of the molecules...
Temp in the upper atmosphere is 900°C but you'd freeze there, as there is no atmosphere to conduct the heat to you.
Interesting fact: Concorde passengers could not touch the windows, not because they were too cold from the altitude, but too hot from the air friction of the plane going 2x the speed of sound.
Thermodynamics: how heat moves around (cold does not move, but heat does)
Three ways heat moves: radiation, conduction, convection
Three laws of thermo:
1. you can't win (nothing has more than 100% efficiency)
2. you can't break even (you always lose something in every reaction, a "heat tax")
3. you can't get out of the game (all reactions tend towards disorder, like your closet)
Efficiency is the amount you get out of any energy reaction, divided by the amount that went in, always less than 100%
efficiency; never 100%, 30-60% common
Human 35% efficiency, diesel engine 60%, Formula one racing car: 55%
Energy "quality" is the degree of organization of the energy (sugar molecules vs. heat coming from your body, or well organized gasoline "octane" molecules breaking into heat, CO2 and H2O)
Entropy: degree of disorder in any system, all reactions tend towards more disorder (e.g. your closet or bedroom-tell this to your parents)
energy quality-entropy, disorder, e.g. closet ∆S>0, takes energy input to reduce S (entropy)
Feedback mechanisms:
Feedback: think of the howling speakers at assembly: microphone picks up the speaker, gets louder, goes on and on: positive feedback
Negative feedback: tends towards stability, e.g. a pendulum: the more you pull it away, the stronger the restore force, e.g. sweating, stable ships at sea
Positive feedback: tends away from stability, e.g. climate change: heat melts ice, dark water absorbs heat, more ice melts, also tall cruise ships, childbirth, blood loss in accidents, albedo decrease in the arctic...
Next: cycles
/groups/apenvironmentalscience/search/index.rss?tag=hotlist/groups/apenvironmentalscience/search/?tag=hotWhat’s HotHotListHot!?tag=hot6/groups/apenvironmentalscience/sidebar/HotListadminadmin2020-08-19 15:43:59+00:002020-08-19 15:43:59updated30adminadmin2011-09-08 21:36:21+00:002011-09-08 21:36:21updated29adminadmin2011-08-24 23:20:40+00:002011-08-24 23:20:40updated28adminadmin2011-08-24 22:42:36+00:002011-08-24 22:42:36updated27adminadmin2011-08-22 02:41:09+00:002011-08-22 02:41:09updated26adminadmin2011-08-22 02:40:02+00:002011-08-22 02:40:02updated25adminadmin2011-08-21 20:39:11+00:002011-08-21 20:39:11updated24adminadmin2011-08-21 20:30:42+00:002011-08-21 20:30:42updated23adminadmin2011-08-21 20:30:13+00:002011-08-21 20:30:13updated22adminadmin2011-08-21 20:25:48+00:002011-08-21 20:25:48updated21adminadmin2011-08-21 20:25:18+00:002011-08-21 20:25:18updated20adminadmin2011-08-21 00:22:12+00:002011-08-21 00:22:12updated19adminadmin2011-08-21 00:18:56+00:002011-08-21 00:18:56updated18adminadmin2011-08-21 00:15:43+00:002011-08-21 00:15:43updated17adminadmin2011-08-21 00:12:37+00:002011-08-21 00:12:37updated16adminadmin2011-08-21 00:12:02+00:002011-08-21 00:12:02updated15adminadmin2011-08-20 23:59:41+00:002011-08-20 23:59:41updated14Added tag - hotadminadmin2011-08-20 23:59:38+00:002011-08-20 23:59:38addTag13Added tag - conservationadminadmin2011-08-20 23:59:32+00:002011-08-20 23:59:32addTag12Added tag - critical thinkingadminadmin2011-08-20 23:59:19+00:002011-08-20 23:59:19addTag11Added tag - ch1adminadmin2011-08-20 23:59:08+00:002011-08-20 23:59:08addTag10Added tag - sustainabilityadminadmin2011-08-20 23:59:05+00:002011-08-20 23:59:05addTag9adminadmin2011-08-20 20:47:39+00:002011-08-20 20:47:39updated8adminadmin2011-08-20 20:46:15+00:002011-08-20 20:46:15updated7adminadmin2011-08-20 20:43:07+00:002011-08-20 20:43:07updated6adminadmin2011-08-20 19:14:13+00:002011-08-20 19:14:13updated5adminadmin2011-08-20 19:11:26+00:002011-08-20 19:11:26updated4adminadmin2011-08-20 18:59:57+00:002011-08-20 18:59:57updated3adminadmin2011-08-20 18:56:59+00:002011-08-20 18:56:59updated2First createdadminadmin2010-11-07 01:41:28+00:002010-11-07 01:41:28created1wiki2020-08-19T15:43:59+00:00groups/apenvironmentalscience/wiki/welcomeFalseCh01 Overview/groups/apenvironmentalscience/wiki/welcome/Ch01_Overview.htmladmin30 updatesCh01 Overview
Welcome to our APES wiki. You should be able to do the following after logging in with your account:
To create a new page, click the ...Falseadmin2020-08-19T15:43:59+00:00adminadmin2013-02-05 02:24:03+00:002013-02-05 02:24:03updated4Added tag - hotadminadmin2013-02-05 02:24:02+00:002013-02-05 02:24:02addTag3adminadmin2013-02-05 02:05:35+00:002013-02-05 02:05:35updated2First createdadminadmin2013-02-05 02:03:35+00:002013-02-05 02:03:35created1wiki2013-02-05T02:24:03+00:00groups/apenvironmentalscience/wiki/394a8FalseEnergy notes/groups/apenvironmentalscience/wiki/394a8/Energy_notes.htmladmin4 updatesEnergy notes
Week of 2.4.13: energy wrap-up
e2 video: coal vs. nuclear in class
AP exams: FRQ
2002.1
2004.2
2006.1
2007.2
2008.1
...Falseadmin2013-02-05T02:24:03+00:00adminadmin2013-02-05 02:23:20+00:002013-02-05 02:23:20updated6Added tag - hotadminadmin2013-02-05 02:23:18+00:002013-02-05 02:23:18addTag5adminadmin2013-02-05 02:23:12+00:002013-02-05 02:23:12updated4adminadmin2013-02-05 02:21:48+00:002013-02-05 02:21:48updated3adminadmin2013-02-05 02:20:26+00:002013-02-05 02:20:26updated2First createdadminadmin2013-02-05 02:06:00+00:002013-02-05 02:06:00created1wiki2013-02-05T02:23:20+00:00groups/apenvironmentalscience/wiki/c360bFalseFeb-May plan/groups/apenvironmentalscience/wiki/c360b/FebMay_plan.htmladmin6 updatesFeb-May plan
1. conclusion of energy chapters (see previous wiki)
2. GCC AP questions FRQ:
2006.2
2005.3
2005.4
2007.3
...Falseadmin2013-02-05T02:23:20+00:00adminadmin2012-03-07 05:53:55+00:002012-03-07 05:53:55updated14adminadmin2012-03-07 05:43:38+00:002012-03-07 05:43:38updated13adminadmin2012-03-07 05:41:35+00:002012-03-07 05:41:35updated12adminadmin2012-03-07 05:38:57+00:002012-03-07 05:38:57updated11Added tag - hotadminadmin2012-03-07 05:38:55+00:002012-03-07 05:38:55addTag10adminadmin2012-03-07 05:36:47+00:002012-03-07 05:36:47updated9adminadmin2012-03-07 05:22:26+00:002012-03-07 05:22:26updated8adminadmin2012-03-07 05:20:01+00:002012-03-07 05:20:01updated7adminadmin2012-03-07 05:18:58+00:002012-03-07 05:18:58updated6adminadmin2012-03-07 04:58:55+00:002012-03-07 04:58:55updated5adminadmin2012-03-07 04:57:33+00:002012-03-07 04:57:33updated4adminadmin2012-03-07 04:56:53+00:002012-03-07 04:56:53updated3adminadmin2012-03-07 04:54:20+00:002012-03-07 04:54:20updated2First createdadminadmin2012-03-07 04:53:33+00:002012-03-07 04:53:33created1weblog2012-03-07T05:53:55+00:00groups/apenvironmentalscience/weblog/de030FalseGreen Apple/groups/apenvironmentalscience/weblog/de030/Green_Apple.htmladmin14 updatesGreen Apple
Team,
Please watch this video about NYC:
Trailer:
http://www.pbs.org/e2/episodes/101_the_green_apple_trailer.html
On the server:
http://physics.hpa...Falseadmin2012-03-07T05:53:55+00:00adminadmin2011-09-13 19:08:24+00:002011-09-13 19:08:24updated4Added tag - hotadminadmin2011-09-13 19:08:22+00:002011-09-13 19:08:22addTag3adminadmin2011-09-13 19:08:10+00:002011-09-13 19:08:10updated2First createdadminadmin2011-09-13 19:04:30+00:002011-09-13 19:04:30created1weblog2011-09-13T19:08:24+00:00groups/apenvironmentalscience/weblog/4ecddFalseQuestions for Wednesday, wiki adds/groups/apenvironmentalscience/weblog/4ecdd/Questions_for_Wednesday_wiki_adds.htmladmin4 updatesQuestions for Wednesday, wiki adds
Team,
I'd like to try something for class tomorrow: each of you to create a question from chapter 3, and email it to me by this evening (Tuesday). Pl...Falseadmin2011-09-13T19:08:24+00:00hot/groups/apenvironmentalscience/search/index.rss?sort=modifiedDate&kind=all&sortDirection=reverse&excludePages=wiki/welcomelist/groups/apenvironmentalscience/search/?sort=modifiedDate&kind=all&sortDirection=reverse&excludePages=wiki/welcomeRecent ChangesRecentChangesListUpdates?sort=modifiedDate&kind=all&sortDirection=reverse&excludePages=wiki/welcome0/groups/apenvironmentalscience/sidebar/RecentChangesListmodifiedDateallRecent ChangesRecentChangesListUpdateswiki/welcomeNo recent changes.reverse5searchlist/groups/apenvironmentalscience/calendar/Upcoming EventsUpcomingEventsListEvents1Getting events…
Comments